Specific Heat Capacity of Copper
NIST Chemistry WebBook The National Institute of. Material Properties - Material properties of.
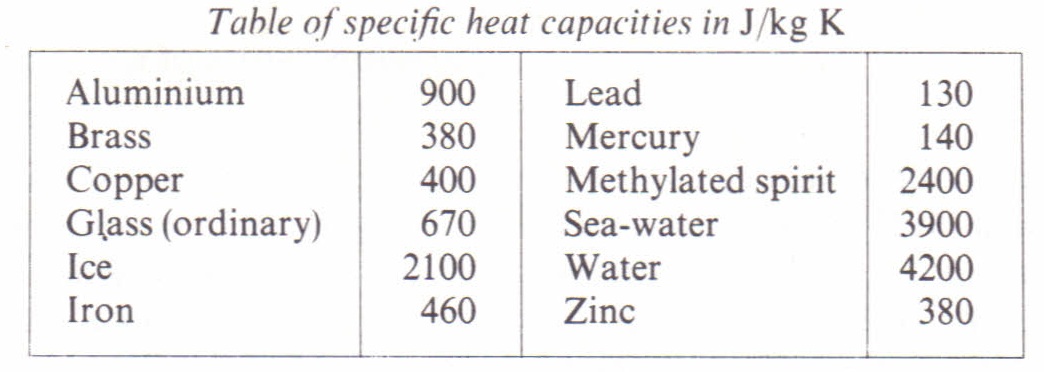
Specific Heat Capacity Physics Homework Help Physics Assignments And Projects Help Assignments Tutors Online
Before the specific heat capacity of copper can be determined it is necessary to know the heat capacity of the calorimeter.

. Q - heat absorbed m - the. How much energy is needed to raise the temperature of 3 kg of copper by 10C. Materials Specific Heat Capacity of Metals Table Chart.
So 1000 H 5 would give the specific heat capacity of copper. The specific heat of carbon steel is 049 kJkgC and the heat required can be calculated as. The solid line shows the values predicted by the model.
I Heat capacity Mass Specifc heat capacity 150 10 3 k g 410 J k g K 615 J K The heat capacity of copper is 615 JK ii QmcΔT where. Download scientific diagram Variation of the specific heat of copper with temperature. Specific Heat Capacity of Chemical Elements.
The specific heat of copper at 20 0C is 00918 cal per gram. Specific heat of copper at temperatures below 0. 71 rows Related Resources.
The heat added to warm or subtracted to cool is given by. Specific heat of copper at temperatures below 0. Symbols show experimental data from Osborne.
2 kg of carbon steel is heated from 20 oC to 100 oC. Please scroll down the compounds are sorted alphabetically. Top Solid Phase Heat Capacity Shomate Equation References Data from NIST Standard Reference Database 69.
Specific heat of Copper is 038 Jg K. Heat capacity is an extensive property of matter meaning it is proportional to the size of the system. The value can be found by performing an experiment with a metal.
Calculate the specific heat capacity of the metal you have been given. Or use the CtrlF. How is molar heat capacity related to.
Specific heat of copper. 390 JKg C specific heat capacity of copper means 390 J of energy is required to raise the temperature of 1 kg of copper by 1 C. The specific heat capacity is defined as the quantity of heat J absorbed per unit mass kg of the material when its temperature increases 1 K or 1 C and.
Heat capacity is the quantity of heat absorbed emitted whole body in the process of heating cooling by 1 Kelvin. The specific heat capacity for copper is 385 JkgC Delta E_t mcDelta theta. Heat capacity C has the unit of energy per degree.
Specific heat is a physical quantity. The difference can come from the temperature at which has been measured the specific heat capacity. Show all of your data and working outList the assumptions you make for this experiment.
Specific heat or specific heat capacity is a property related to internal energy that is very important in thermodynamics. The specific heat capacity of solid copper metal is. If 226 kJ of heat increases the temperature of 470 kg of copper by 125 degrees Celsius what is the molar heat capacity of copper.
The specific heat of water is 100 calgoC. From the given information The specific. Q m c T f - T i or Q mc T The value of c al for aluminum is 0215calg oC.

Can Anyone Suggest A Material With The Highest Specific Heat Capacity Higher Than Water

Find Specific Heat Physics Help Physical Properties Of Matter Physics

Specific Heat Of Water Very High So It Takes More Energy To Increase The Temperature Of A Given Mass Of Wat Teaching Science Science Facts Homeschool Science

Solved Table 9 1 Specific Heat Capacity For Different Chegg Com
0 Response to "Specific Heat Capacity of Copper"
Post a Comment